Alagille Syndrome: Genetic Disease That Affects The Liver And Heart
Alagille syndrome is also known as Alagille-Watson syndrome or arteriohepatic dysplasia. It is a genetic disease that affects the heart, liver, and other organs. The first symptoms appear during childhood.
This disease was described for the first time in 1969 by the French pediatrician Daniel Alagille. It is currently estimated that it affects 1 in every 70,000 children born. However, some authors consider that its prevalence may be 1 in every 30,000 births.
The level of severity of Alagille syndrome is highly variable. While in some patients the symptoms go unnoticed, in others it generates serious health conditions, which can lead to a heart or liver transplant.
What is Alagille Syndrome?
Alagille syndrome is a disease that affects several organs. Among them, the heart, liver, spine, face, eyes, blood vessels and kidneys. At first it was called syndromic shortage of the bile ducts.
This disorder is caused by a mutation in a gene called JAGGED1 or the NOTCH2 gene. Up to 94% of cases correspond to the JAGGED1 mutation, while only between 1 and 2% are related to NOTCH2. In all other cases, the cause is unknown.
What is most characteristic of Alagille syndrome is that patients have liver diseases that are caused by a decrease in the number of bile ducts.
Causes
The JAGGED1 gene is located on chromosome 20. It controls the formation of a protein called JAG1. For its part, the NOTCH2 gene is on chromosome 1. When the mutation occurs in these genes, the cell nucleus is altered.
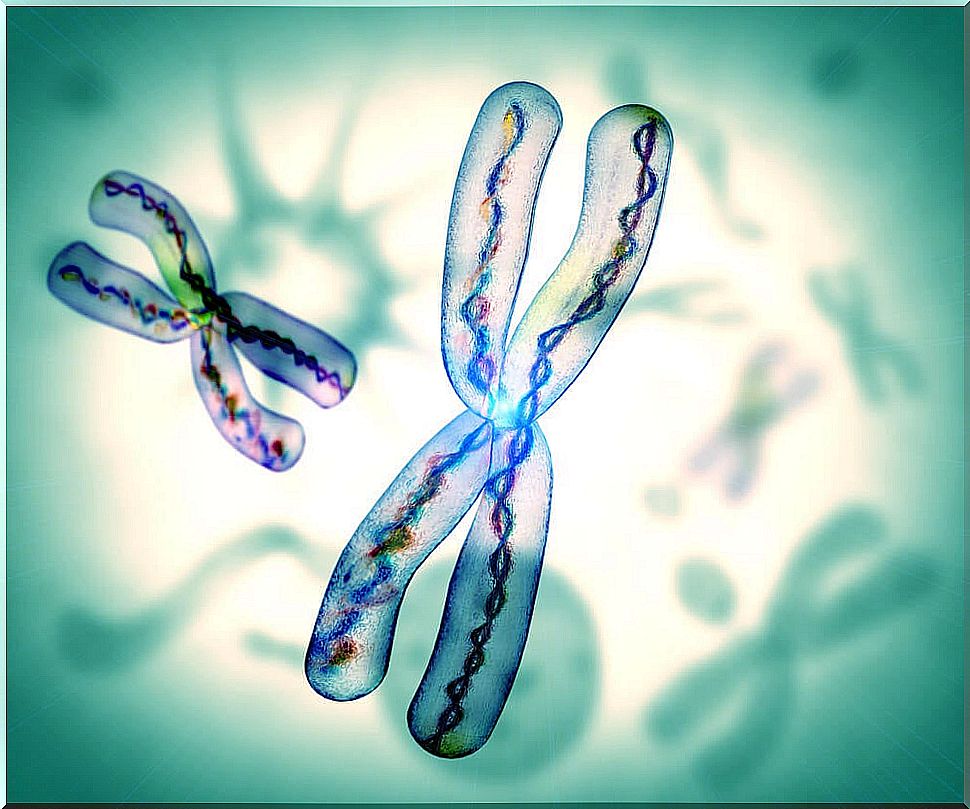
All this leads to the activation of other genes that directly affect the growth and development of various organs of the body. Mainly from the brain, liver, skeleton, and kidneys. Science currently does not know why the mutation generates these effects.
Alagille syndrome is hereditary dominant. When one of the parents has the disease, they also have a normal gene and a mutated one. Therefore, your children have a 50% chance of inheriting the disease. In 20% of cases the syndrome is caused by a new mutation.
Clinical features
Thanks to the investigations carried out since 1969, it has been possible to establish that five basic abnormalities are present in Alagille syndrome. This set of anomalies is called “classical criterion” and from this the diagnosis is made. The components of the classical criterion are:
- Liver disease. It is present in approximately 95% of cases.
- Heart disease. It occurs in about 90% of affected patients.
- Ophthalmological manifestations. They are present in a percentage ranging from 76% to 95% of patients.
- Skeletal abnormalities. In about 80% of patients a conformation of the vertebrae called “butterfly wings” is observed.
- Specific facial features. They are mild, but recognizable. They basically include a prominent forehead, sunken eyes, a straight nose with a bulbous tip, etc.
Vascular involvement, kidney involvement, growth retardation, pancreatic involvement, and learning difficulties are also common.
Decreased number of bile ducts is considered a fundamental pattern in Alagille syndrome. Typical symptoms include jaundice, eye spots, and xanthelasmas (small fatty growths around the eyelids).
Diagnosis and prognosis
Alagille syndrome is diagnosed by a thorough clinical examination, blood tests, and other special tests. The usual thing is that when there is suspicion of the disease, a blood test and a urine test are ordered, in principle.
These tests measure the amount of bilirubin in the blood. They also offer information on the functioning of the liver and kidneys. An ultrasound of the liver and kidneys and a liver biopsy are also usually ordered.
The liver biopsy is done with a needle and allows to know if there is a shortage of bile ducts. X-rays, heart tests, and eye tests are also routinely done. The gene mutation can be detected by a blood test.
Most patients with Alagille syndrome can lead almost normal lives. Only if there are severe heart or liver problems can life expectancy be shortened.